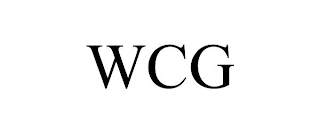
| Serial Number | 97922801 |
| Word Mark | WCG |
| Filing Date | Friday, May 5, 2023 |
| Status | 748 - STATEMENT OF USE - TO EXAMINER |
| Status Date | Tuesday, June 10, 2025 |
| Registration Number | 0000000 |
| Registration Date | NOT AVAILABLE |
| Mark Drawing | 4 - Illustration: Drawing with word(s) / letter(s) / number(s) in Block form |
| Published for Opposition Date | Tuesday, October 29, 2024 |
| Goods and Services | Medical evaluation of and medical diagnostic services for potential clinical trial patients with arthritic-based issues; medical evaluation and medical diagnostic testing, monitoring and reporting services of patients with arthritic-based issues for potential participation in clinical trials; providing an on-line database featuring healthcare research information |
| Goods and Services | financial management of reimbursement payment for other clinical trial sites; financial management in the field of clinical trials, namely, accounts receivables financing and accounts payable debiting, for clinical trial sites; executing and tracking payments from the research sponsor to its associated research sites for payment for research studies; Financial advisory services, namely, budget negotiation in the field of clinical trials; Financial advisory services, namely, budget negotiation in the field of clinical trials, namely, between biopharmaceutical companies, contract research organizations and investigator sites around the world |
| Goods and Services | providing business administrative procedure services for obtaining descriptions of participants' consents in clinical trials; business assistance for administrative procedures with schedule management of examination participants' visits and examinations, clinical investigators, and clinical participants; providing business administrative assistance for the creation and preservation of clinical trial documents and records related to the trial implementation plan, case report, substitute for recruitment of subjects; Business consulting, management, and planning services in the field of clinical trials, namely, cost analyses of patient insurance coverage for businesses, negotiating business contracts on behalf of research sites; business consulting, management, and planning services in the field of clinical trials, namely, analyzing and reporting possible cost savings in human research study protocol development and possible cost savings in research site identification on behalf of pharmaceutical trial sponsors and contract research organizations, all for business cost management purposes; business consulting, management, and planning services in the field of clinical trials, namely, applying business analytics to determine the appropriate payer coverage and for use in assisting with study budget negotiations for clinical trial sites; business consulting in the field of clinical trial study identification on behalf of clinical trial sites, namely, negotiating business contracts on behalf of clinical trial sites; business consulting in the field of clinical trials, namely, negotiating business contracts on behalf of clinical trial sites; business consulting and management in the field of clinical trials, namely, providing information management services; Business services, namely, formulation of best practices for clinical trial execution and management for the testing of drugs, biologics, and medical devices; management of telephone call centers for others; preparation of business reports related to key trends and innovations in clinical trial execution and management for the testing of drugs, biologics, and medical devices; Professional staffing services and staff management of institutional review boards, institutional biosafety committees and conflicts of interest; providing business administrative assistance to the officials within the research site who oversee the human subjects research; negotiating clinical trial business contracts between biopharmaceutical companies, contract research organizations and investigator sites around the world; Administration, billing and reconciliation of accounts on behalf of other clinical trial sites; providing patient retention services for retaining patients in clinical trials; Management and compilation of secure computerised databases of patient medical information |
| Goods and Services | Training services in the field of clinical trials; training in the field of pharmaceutical testing relating to training on tested protocols designed to evaluate the abuse potential of pharmaceuticals during clinical testing or general clinical practice; Medical training of clinical professionals; training services to be delivered to participants involved in conducting clinical trials in the medical and pharmaceutical industries; Educational services, namely, providing online learning management courses and highly-specialized training for research professionals in the field of clinical trial research training and compliance; arranging and conducting seminars to prospective clinical trial participants about clinical trials research; providing safety instruction in the field of scientific clinical trials to the officials within the research site who oversee the human subjects during research; staff training, namely, business training for others in the field of clinical trial research |
| Goods and Services | Providing independent review of clinical trials involving human subjects, namely, reviewing research protocols and related information to ensure protection of the rights and welfare of human subjects of research; Regulatory compliance consulting in the field of clinical trials and life sciences research, namely, providing consultation to develop a corrective action plan for research misconduct or issues of noncompliance; legal assistance in the drawing up of global contracts for others for resource placement, supported by technology-driven processes and a network of international attorneys who possess specialized, in-depth knowledge of local languages and regulations; Legal assistance in the drawing up of contracts and legal services in relation to the negotiation of contracts for others, all supported by technology-driven processes and an international network of attorneys who know about specific, in-depth local and regulations; Consulting services in the field of international and federal regulatory compliance requirements and guidelines for personnel safety relating to the protection of the rights and welfare of human subjects of clinical research studies |
| Goods and Services | Consulting services for others in the field of implementation project management of testing research protocols designed to evaluate the abuse potential of pharmaceuticals during clinical testing or general clinical practice and the analysis of those results; clinical research consulting and advisory services related to ensuring the safety of pharmaceutical preparations administered to patients during clinical trials; collecting and providing statistical analysis of clinical trial data for clinical research purposes; technical consulting in the field of clinical research trials for the testing of drugs, biologics, and medical devices; providing information on drug clinical trials; Providing online non-downloadable computer software to manage health care research and support information through the Internet; providing temporary use of online non-downloadable software for use in assisting clinical trial sites, namely, providing temporary use of online non-downloadable software for conducting informed patient consent processes and featuring technology that enables potential patient users to access consistent institutional review board (IRB) and clinical trial sponsor approved messages, which helps potential patients make informed decisions regarding their participation in clinical studies; Providing a web site featuring technology enabling internet users to manage and exchange healthcare research information; providing consultation in the development and maintenance of standards required to meet accreditation by others in the field of clinical trials involving human subjects and research protocols relating to same; Scientific study and research in the field of clinical research, namely, providing scientific review of human subjects research protocols as to the design and risks involved; providing computer services in support of clinical trial start-up and management, namely, developing and hosting a web-accessed technology platform used for facilitating communication between clinical trial sponsors, contract research organizations, and clinical trial sites; providing a website featuring technology that enables users to collect, manage and share documents to facilitate communication during clinical trials; Providing temporary use of on-line non-downloadable software for conducting assessment surveys regarding suitability for site participation in clinical trials and utilizing data analytics to select appropriate clinical trial site locations; providing online non-downloadable software for posting, tracking, and accessing reports related to clinical training courses attended by clinical trial clinicians; providing scientific research information services, in the field of clinical trials, to prospective or current clinical trial participants, all for the purpose of increasing participant engagement and retention in clinical trials, and provided via telephone and e-mail; providing scientific research information services, in the field of clinical trials, to prospective or current clinical trial participants, all for clinical assistance and quality assurance purposes, and to increase rater reliability in trials where subjective scales are relied upon to improve rating consistency and trial efficacy; Providing on-line non-downloadable software for executing and tracking payments from the research sponsor to its associated research sites for payment for research studies; scientific research in the nature of evaluation and monitoring, namely, assisting and facilitating clinical trials for others; Graphic design of recruitment advertising materials for others for clinical trials; Implementing and managing enterprise system software applications in the nature of Clinical Trial Management System (CTMS) |
| International Class | 035 - Advertising; business management; business administration; office functions. |
| US Class Codes | 100, 101, 102 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 035 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| International Class | 036 - Insurance; financial affairs; monetary affairs; real estate affairs. |
| US Class Codes | 100, 101, 102 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 036 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| International Class | 041 - Education; providing of training; entertainment; sporting and cultural activities. |
| US Class Codes | 100, 101, 107 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 041 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| International Class | 042 - Scientific and technological services and research and design relating thereto; industrial analysis and research services; design and development of computer hardware and software. |
| US Class Codes | 100, 101 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 042 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| International Class | 044 - Medical services; veterinary services; hygienic and beauty care for human beings or animals; agriculture, horticulture and forestry services. |
| US Class Codes | 100, 101 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 044 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| International Class | 045 - Legal services; security services for the protection of property and individuals; personal and social services rendered by others to meet the needs of individuals. |
| US Class Codes | 100, 101 |
| Class Status Code | 6 - Active |
| Class Status Date | Monday, June 5, 2023 |
| Primary Code | 045 |
| First Use Anywhere Date | Monday, June 12, 2023 |
| First Use In Commerce Date | Monday, June 12, 2023 |
| Party Name | WCG Clinical Inc. |
| Party Type | 20 - Owner at Publication |
| Legal Entity Type | 03 - Corporation |
| Address | Princeton, NJ 08540 |
| Party Name | WCG Clinical Inc. |
| Party Type | 10 - Original Applicant |
| Legal Entity Type | 03 - Corporation |
| Address | Princeton, NJ 08540 |
| Event Date | Event Description |
| Tuesday, May 9, 2023 | NEW APPLICATION ENTERED |
| Monday, June 5, 2023 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED |
| Wednesday, January 31, 2024 | ASSIGNED TO EXAMINER |
| Friday, February 9, 2024 | NON-FINAL ACTION WRITTEN |
| Friday, February 9, 2024 | NON-FINAL ACTION E-MAILED |
| Friday, February 9, 2024 | NOTIFICATION OF NON-FINAL ACTION E-MAILED |
| Wednesday, April 24, 2024 | APPLICATION EXTENSION TO RESPONSE PERIOD - RECEIVED |
| Wednesday, April 24, 2024 | APPLICATION EXTENSION GRANTED/RECEIPT PROVIDED |
| Thursday, June 20, 2024 | ASSIGNMENT OF OWNERSHIP NOT UPDATED AUTOMATICALLY |
| Tuesday, July 30, 2024 | TEAS RESPONSE TO OFFICE ACTION RECEIVED |
| Tuesday, July 30, 2024 | CORRESPONDENCE RECEIVED IN LAW OFFICE |
| Tuesday, July 30, 2024 | TEAS/EMAIL CORRESPONDENCE ENTERED |
| Saturday, September 21, 2024 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| Wednesday, October 9, 2024 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| Tuesday, October 29, 2024 | PUBLISHED FOR OPPOSITION |
| Tuesday, October 29, 2024 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |
| Tuesday, December 10, 2024 | NOA E-MAILED - SOU REQUIRED FROM APPLICANT |
| Tuesday, June 10, 2025 | SOU TEAS EXTENSION RECEIVED |
| Tuesday, June 10, 2025 | SOU EXTENSION 1 FILED |
| Tuesday, June 10, 2025 | SOU EXTENSION 1 GRANTED |
| Tuesday, June 10, 2025 | TEAS STATEMENT OF USE RECEIVED |
| Monday, September 15, 2025 | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED |
| Tuesday, June 10, 2025 | USE AMENDMENT FILED |
| Tuesday, October 7, 2025 | STATEMENT OF USE PROCESSING COMPLETE |