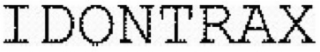Trademark Overview
On Monday, October 20, 2025, a trademark application was filed for IDONTRAX with the United States Patent and Trademark Office. The USPTO has given the IDONTRAX trademark a serial number of 79438708. The federal status of this trademark filing is NEW APPLICATION - RECORD INITIALIZED NOT ASSIGNED TO EXAMINER as of Thursday, December 11, 2025. This trademark is owned by ID SOLUTIONS. The IDONTRAX trademark is filed in the Chemical Products, Pharmaceutical Products, and Medical Instrument Products categories with the following description:
Apparatus, tools and instruments for medical diagnosis; DNA and RNA testing apparatus for medical use; genetic testing apparatus for medical use; quantitative polymerase chain reaction [PCR] instrument for medical use; samplers for medical use.
Diagnostic products, preparations and reagents other than for medical use; screening agents for in-vitro diagnosis for scientific use; diagnostic reagents other than for medical use sold in kit form; diagnostic reagents for in-vitro use other than for medical use; reagents for in vitro laboratory uses other than for medical use; chemical preparations intended for use in DNA analysis, other than for medical use; enzymes, other than for medical use, for continuous, homogeneous and isothermal amplification processes for DNA-specific models; chemical genetic identity tests comprised of reagents; reagents for nucleic acid amplification other than for medical use.
Pharmaceutical products; products, preparations and reagents for diagnosis for medical or veterinary use; diagnostic materials; reagents for medical use; chemical reagents for medical or veterinary use; organic reagents for medical or veterinary use; reagents for analysis for medical use; reagents for diagnostic tests for medical use; reagents for genetic testing in medicine; reagents for in vitro use in laboratories for medical use; in vitro diagnostic preparation for medical use; indicators for medical diagnosis; diagnostic substances for medical use; diagnostic biomarking reagents for medical use; diagnostic test kits for detecting gene mutations implicated in cancers; in vitro test kits for detecting genetic defects; chemical preparations intended for use in DNA analysis for medical use; genetic identity tests comprising reagents for medical use; preparations for detecting genetic predispositions for medical use; reagents for nucleic acid amplification for medical use; diagnostic m...