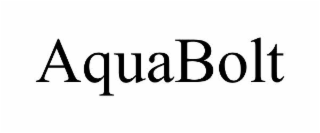
| Serial Number | 99407993 |
| Word Mark | AQUABOLT |
| Filing Date | Tuesday, September 23, 2025 |
| Status | 686 - PUBLISHED FOR OPPOSITION |
| Status Date | Tuesday, January 27, 2026 |
| Registration Number | 0000000 |
| Registration Date | NOT AVAILABLE |
| Mark Drawing | 4 - Illustration: Drawing with word(s) / letter(s) / number(s) in Block form |
| Published for Opposition Date | Tuesday, January 27, 2026 |
| Goods and Services | Probiotic supplements; Homeopathic supplements; Mineral supplements; Herbal supplements; Vitamin supplements; Calcium supplements; Dietary supplements; Food supplements; Prebiotic supplements; Protein supplements; Nutritional supplements; Dietary food supplements; Enzyme food supplements; Propolis dietary supplements; Liquid herbal supplements; Weight management supplements; Health food supplements; Whey protein supplements; Protein dietary supplements; Liquid protein supplements; Casein dietary supplements; Yeast dietary supplements; Albumin dietary supplements; Natural dietary supplements; Alginate dietary supplements; Flaxseed dietary supplements; Linseed dietary supplements; Vegan dietary supplements; Vegan food supplements; Vegan nutritional supplements; Vegan protein supplements; Pollen dietary supplements; Chlorella dietary supplements; Liquid vitamin supplements; Zinc dietary supplements; Mineral food supplements; Lutein dietary supplements; Fenugreek dietary supplements; Soy protein dietary supplements; Royal jelly dietary supplements; Vitamin and mineral supplements; Vegan liquid protein supplements; Dietary and nutritional supplements; Pine pollen dietary supplements; Dietary supplements for animals; Vegan dietary food supplements; Activated charcoal dietary supplements; Linseed oil dietary supplements; Protein supplements for animals; Flaxseed oil dietary supplements; Sea moss dietary supplements; Whey protein dietary supplements; Dietary supplements for controlling cholesterol; Dietary supplements for pets; Dietary supplements for humans; Brewer's yeast dietary supplements; Coenzyme Q10 dietary supplements; Natural supplements for treating candida; Dietary supplements for human consumption; Ketogenic dietary and nutritional supplements; Vegan dietary supplements for animals; Vegan dietary supplements for pets; Vegan protein supplements for animals; Food supplements for veterinary purposes; Food supplements for veterinary use; DHA algae oil dietary supplements; Dietary supplements for human beings; Folic acid dietary supplements; Dietary supplements for urinary health; Herbal supplements for sleeping problems; Natural supplements for treating erectile dysfunction; Dietary supplements for treatment of claustrophobia; Medicated supplements for foodstuffs for babies; Dietary supplements with a cosmetic effect; Ganoderma lucidum spore powder dietary supplements; Medicated supplements for foodstuffs for animals; Dietary supplements for humans and animals; Dietary supplements consisting primarily of iron; Nutritional supplements consisting primarily of iron; Dietary supplements containing reishi mushroom powder; Vegan food supplements for veterinary use; Dietetic supplements adapted for medical purposes; Natural supplements for treating depression and anxiety; Vitamins and dietary food supplements for animals; Dietary and nutritional supplements for endurance sports; Nutritional supplements in capsule form for dogs; Dietary supplements for human beings and animals; Dietary supplements containing lion's mane mushroom powder; Dietary and nutritional supplements used for weight loss; Animal feed additives for use as nutritional supplements; Vegan protein supplements formed and packaged as bars; Protein dietary supplements formed and packaged as bars; Dietary pet supplements in the form of pet treats; Nutritional and dietary supplements formed and packaged as bars; Dietary supplements in the nature of weight loss powders; Ketogenic dietary and nutritional supplements used for weight loss; Vegan protein dietary supplements formed and packaged as bars; Vegan dietary and nutritional supplements used for weight loss; Nutritional supplements in the nature of nutritionally fortified soft chews; Green coffee bean extracts for use as dietary supplements; Alkalinity buffer supplements for live coral for use in aquariums; Calcium-based nutrient supplements for live coral for use in aquariums; Non-medicated additives for animal feed for use as nutritional supplements; Dietary supplements for pets in the nature of a powdered drink mix; Fungal extracts sold as a component ingredient of nutritional supplements and vitamins; Dietary fiber for use as an ingredient in the manufacture of dietary supplements; Dietary beverage supplements for human consumption in liquid and dry mix form for therapeutic purposes; Dietary and nutritional supplements containing creatine; Protein supplement shakes; Nutritional supplement shakes; Liquid nutritional supplement; Zinc supplement lozenges; Vitamin supplement patches; Nutritional supplement energy bars; Powdered nutritional supplement concentrate; Dietary supplement drink mixes; Food supplements, namely, anti-oxidants; Nutritional supplements, namely, probiotic compositions; Vegan powdered nutritional supplement drink mix; Lecithin for use as a dietary supplement; Powdered fruit-flavored dietary supplement drink mix; Wheatgrass for use as a dietary supplement; Powdered nutritional supplement drink mix and concentrate; Nutritional supplements, namely, carbohydrates in powdered form; Wheat for use as a dietary supplement; Nutraceuticals for use as a dietary supplement; Protein supplement shakes for weight gain purposes; Flavonoids for use as a dietary supplement; Powdered nutritional supplement drink mix; Reishi mushroom powder for use as a dietary supplement; Lion's mane mushroom powder for use as a dietary supplement; Animal feed additive for use as a nutritional supplement for medical purposes; Powdered nutritional supplement drink mix containing electrolytes; Soy protein for use as a nutritional supplement in various powdered and ready-to-drink beverages; Vitamin supplement in tablet form for use in making an effervescent beverage when added to water; Calcium montmorillonite clay for therapeutic purposes used to enhance the production of enzymes in living beings or as a mineral supplement; Nutraceuticals for use as a dietary supplement for brain function |
| International Class | 005 - Pharmaceutical and veterinary preparations; sanitary preparations for medical purposes; dietetic substances adapted for medical use, food for babies; plasters, materials for dressings; material for stopping teeth, dental wax; disinfectants; preparations for destroying vermin; fungicides, herbicides. |
| US Class Codes | 005, 006, 018, 044, 046, 051, 052 |
| Class Status Code | 6 - Active |
| Class Status Date | Tuesday, September 23, 2025 |
| Primary Code | 005 |
| First Use Anywhere Date | NOT AVAILABLE |
| First Use In Commerce Date | NOT AVAILABLE |
| Party Name | Advantage Products Group LLC |
| Party Type | 20 - Owner at Publication |
| Legal Entity Type | 16 - Limited Liability Company |
| Address | Prescott, AZ 86305 |
| Party Name | Advantage Products Group LLC |
| Party Type | 10 - Original Applicant |
| Legal Entity Type | 16 - Limited Liability Company |
| Address | Prescott, AZ 86305 |
| Event Date | Event Description |
| Tuesday, September 23, 2025 | NEW APPLICATION ENTERED |
| Tuesday, September 23, 2025 | APPLICATION FILING RECEIPT MAILED |
| Tuesday, September 23, 2025 | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED |
| Wednesday, December 31, 2025 | ASSIGNED TO EXAMINER |
| Wednesday, December 31, 2025 | APPROVED FOR PUB - PRINCIPAL REGISTER |
| Wednesday, January 21, 2026 | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED |
| Tuesday, January 27, 2026 | PUBLISHED FOR OPPOSITION |
| Tuesday, January 27, 2026 | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED |